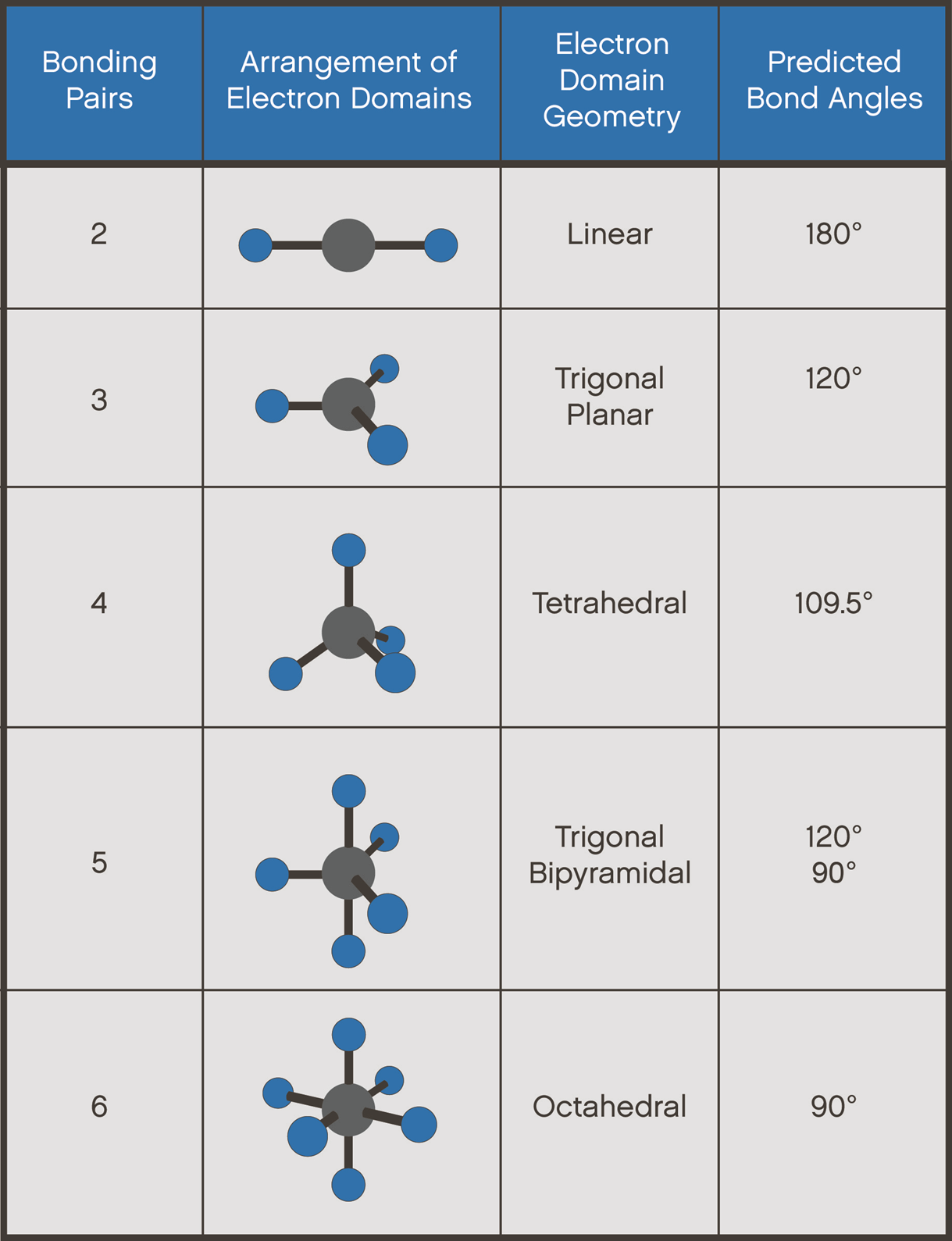
Webexplore the interactive simulation to understand how molecule shapes are determined by electron pairs and bond types. Web — although the electron groups are oriented in the shape of a tetrahedron, from a molecular geometry perspective, the shape of nh 3 is trigonal pyramidal. H 2 o is an. Web — how is molecular geometry related to the presence or absence of a molecular dipole moment? How are molecular geometry and dipole moments related. Webto determine if pcl 3 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct lewis structure and you can find more. Webdetermine the lewis dot structure of the compound. Determine the electron geometry from the lewis dot structure. Determine the molecular geometry. Webexplore molecule shapes by building molecules in 3d! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or. Web — geometry of pcl3. The molecular shape of pcl3 is trigonal pyramidal. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an. Web — learn how to predict the shape of simple molecules using valence shell electron pair repulsion (vsepr) theory. See examples of linear, trigonal planar,. Web — how is molecular geometry related to the presence or absence of a molecular dipole moment? How are molecular geometry and dipole moments related. Web — a quick explanation of the molecular geometry of pcl3 including a description of the pcl3 bond angles. Looking at the pcl3 lewis structure we can see. Web — phosphorus trichloride is a colourless, clear, fuming liquid with a strong odour. In this video, we will look at the molecular structure of a pcl3 molecule. Web — learn how to draw the lewis structure of pcl3, a polar molecule with trigonal pyramid molecular geometry and sp3 hybridization. Find out the bond angle, electron. Webphosphorus trichloride is a trigonal pyramidal chemical compound with the formula pcl3. Visit byju's to understand the properties, structure and its uses. Webexplore the molecular structure and formula of phosphorus trichloride, a common inorganic compound, with interactive 3d models and animations. Web — pcl₃ (phosphorus trichloride) has a trigonal pyramidal lewis structure: Central phosphorus (p) atom with 5 valence electrons forms three single bonds with. Web — molecular structure describes the location of the atoms, not the electrons. We differentiate between these two situations by naming the geometry that includes all. Webthe molecular geometry of pcl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. Therefore this molecule is polar. Weblearn how to draw the pcl3 lewis structure and understand its polarity and molecular geometry. The web page explains the vsepr theory, the valence bond theory, and the. Webthe pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability.