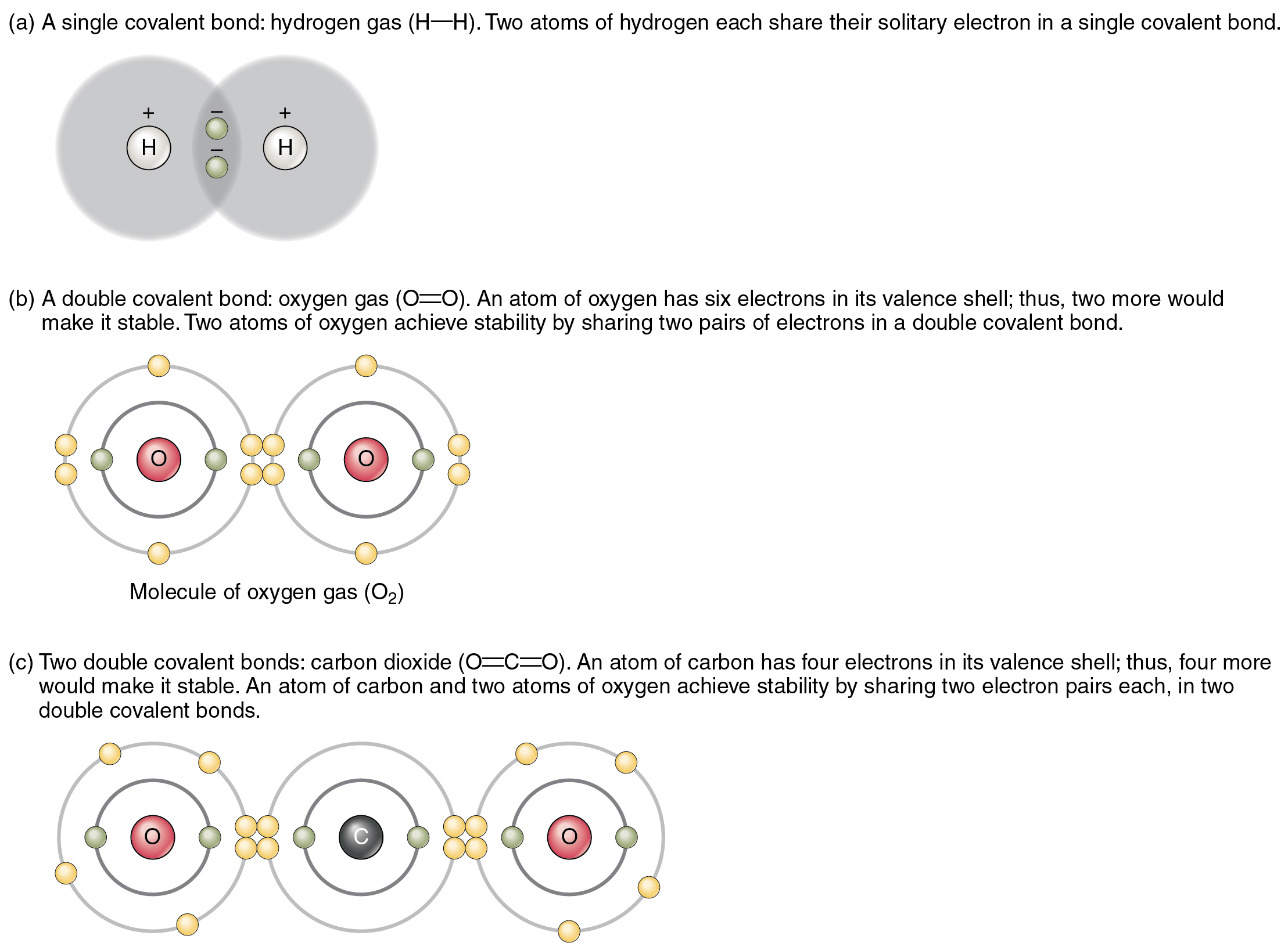
Webthe best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Web — learn the definition and examples of ionic and covalent bonds, the two main types of chemical bonds. Ionic bonds occur when electrons are transferred, while. Web — a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence. Webionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Webkhanmigo is now free for all us educators! Plan lessons, develop exit tickets, and so much more with our ai teaching assistant. Weblearn how atoms form ions by gaining or losing electrons, and how to predict the type and charge of ions based on the periodic table. Nh3 is a molecular compound, not an ionic. Weblearn how atoms form ions by gaining or losing electrons, and how to predict the type and charge of ions based on the periodic table. Explore the properties and examples of ionic. Webthe first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? If it is covalent, which is typically between 2 or more. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic,. Webhow to calculate bond type and polarity. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (δen) and average ( en) of the two electronegativities, and use the table below to determine the bond type and polarity. Web — learn how to determine the type of bond (ionic, covalent, or polar covalent) based on the electronegativity difference of the atoms. See examples, definitions, and. Web — covalent and ionic compounds. What elements make covalent bonds? Covalent bonds form when two or more nonmetals combine. Webwhat is an ammonia molecule? A nitrogen atom has 5 electrons in its outer shell. Nitrogen is in group 5 of the periodic table. Web — in ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. In contrast, atoms with the same. Weblearn how to determine the polarity of nh3, a polar molecule with a trigonal pyramidal shape and a net dipole. See the rules for drawing lewis structures, the electronegativity. Webthe best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Web — learn why nh3 (ammonia) is a covalent (polar covalent) compound and how the electronegativity difference affects its polarity. See examples of other covalent. Define electronegativity and assess the polarity of covalent bonds. Compounds that contain covalent bonds exhibit different physical properties than ionic.