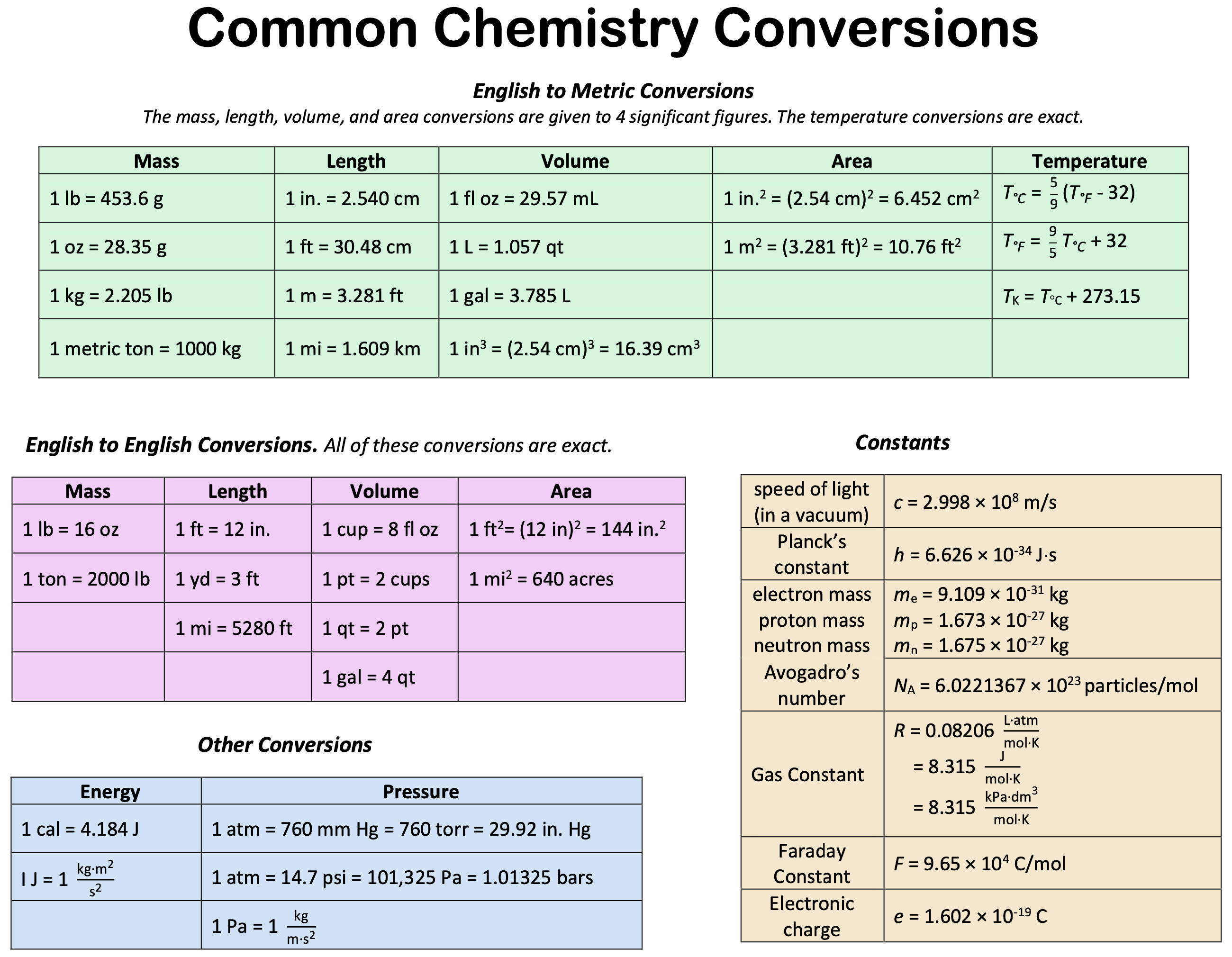
Formula unit helps us in giving an empirical formula that can be used for understanding the compound. It is the unit that represents an entire compound. Formula units refer to the simplest collection of atoms from which an ionic compound’s formula can be established. It represents the ratio between cations (positively charged ions). [ 1 ] [ 2 ] it can also refer to the chemical formula. A formula unit is the simplest formula of an ionic compound and therefore you can think of it as the empirical formula of an ionic compound. But since salts are a repeating solid. How to determine a formula is molecule or formula unit? I read difference sources but still confuse on how to determine a formula is a. Explain the meaning of the formula of an ionic solid such as nacl. Define molecular weight, formula weight, and molar mass. Calculate any of these from any chemical. As indicated in the introduction, this form of characteristic equation appears. Formula mass unit example. Formula mass units can be formulated by adding the mass of the number of moles of the atom present in the compound. This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science. The mole is an amount unit. There are different ways to measure weight, distance, and temperature. How do you find the formula of an ionic compound from its name or vice versa? Watch this worked example video to learn how to apply the rules of naming and writing ionic formulas. The molecular mass and the formula mass of a compound are obtained by adding together the atomic masses of the atoms present in the molecular formula or empirical. Write the formula for a formula unit of iron (iii) carbonate. Solution the symbol for iron is fe. The formula for carbonate is co_3. The charge on iron (iii) is +3, so. Calculating formula units can help you understand the structure and composition of a compound, which is useful in various chemical applications. In this article, we will guide you. Dimensional analysis is shared under a license and was authored, remixed, and/or curated by yogita kumari. Dimensional analysis is used in. The formula unit is the absolute grouping of atoms or ions represented by the empirical formula of a compound, either ionic or covalent. Butane, for example, has the empirical formula c 2 h 5,. The molar mass of a substance is the sum of the average molar masses of the atoms that compose the substance. The molar mass of a substance can be used as a. As a definition formula unit in chemistry is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. In the world of chemistry, understanding the composition of compounds is essential. One fundamental concept in this regard is the. The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units.