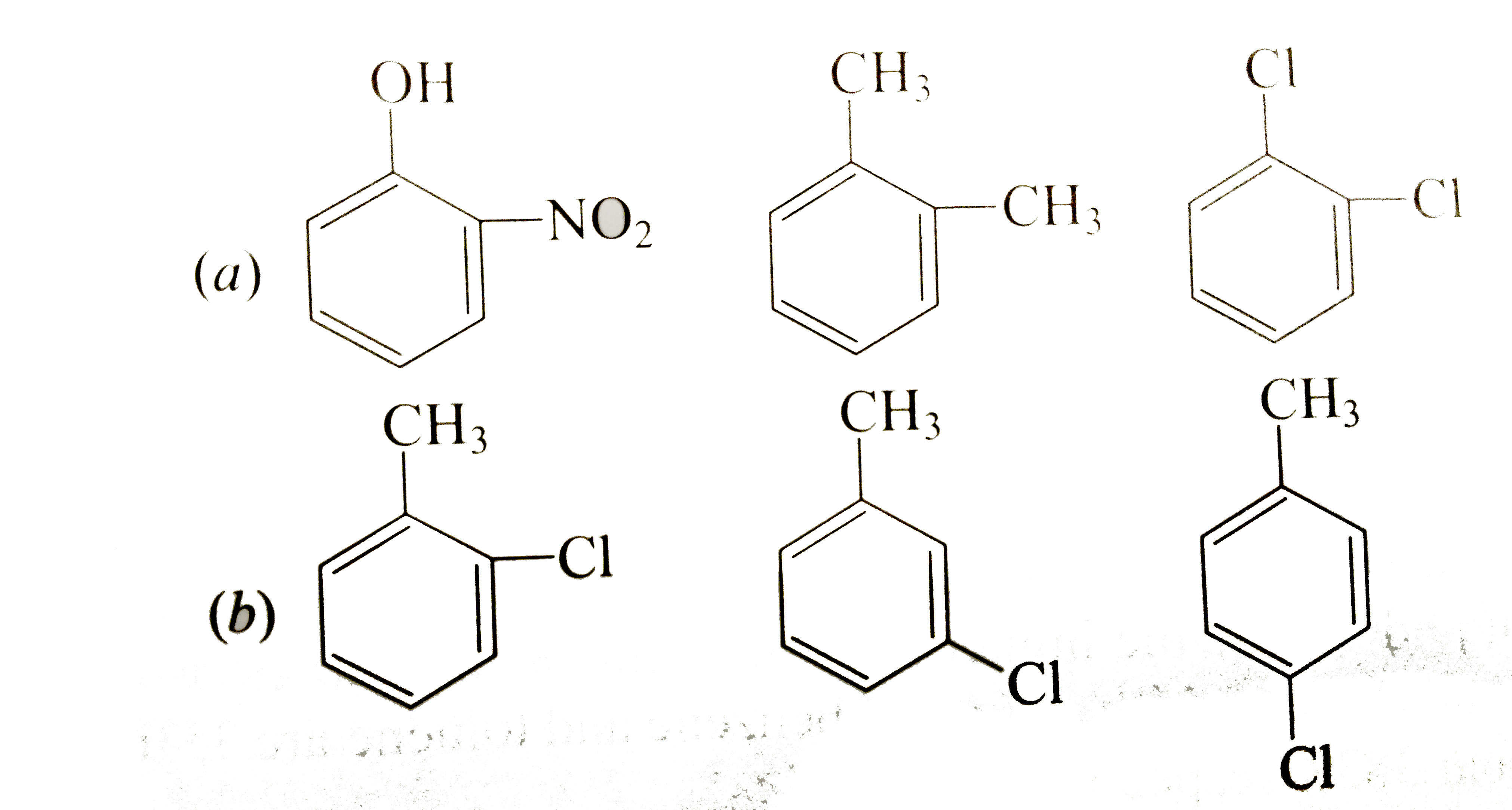
Choose the one alternative that best completes the statement or answers the question. Based on molecular mass and dipole moment of the. Webdipole moments tell us about the charge separation in a molecule. The larger the difference in electronegativities of bonded atoms, the larger the dipole moment. Webdipole moments occur when there is a separation of charge. They can occur between two ions in an ionic bond or between atoms in a covalent bond; The correct option is ch₃cn. Ch₃och₃ is a polar molecule due to the presence of electronegative atom oxygen (o). The geometry will be tetrahedral. The order of strengths of intermolecular forces is: Compounds with stronger intermolecular. Webchrome_reader_mode enter reader mode { readings_i : Property get [map mindtouch. deki. logic. extensionprocessorqueryprovider+c__displayclass230_0. Weball substances experience dispersion forces between their particles. Substances with covalent bonds between. Webanother factor that determines the polarity of a compound is dipole moment, which is the magnitude of the product of the partial charge of atoms and the distance. Webidentify the compound with the highest dipole moment. A) ch3och3 b) ch3ch2ch3 c) ch3cho d) (ch2)2o e) ch3cn. Your solution’s ready to go! Our expert help has. Weba dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. It is denoted by the greek letter ‘µ’. Molecular geometry or shape. Now, let us discuss the effect of the above three factors one by one to prove that the dimethyl ether (ch3och3). ) identify the compound with the lowest dipole moment. Webdipole moments occur when there is a separation of charge. They can occur between two ions in an ionic bond or between atoms in a covalent bond; Webthe electric dipole moment is a measure of the charge distribution in a molecule. The calculated dipole moments are reported as an unsigned total dipole and as three. Webindividual bond dipole moments are indicated in black.